Focal One
Project Description
The treatment of localized prostate cancer by focused ultrasound is a minimally invasive procedure that has been developed since 1990 thanks to a collaboration between 4 partners all based in Lyon, France : the department of urology and transplantation surgery and the department of urinary radiology both at the Edouard Herriot Hospital (Hospices Civils de Lyon), the INSERM unit 1032 (Laboratory of Therapeutic Applications of Ultrasound, LabTAU) University of Lyon 1, and the company EDAP-TMS. The aim was to develop a treatment as minimally-invasive as possible of localized prostate cancer, made necessary by the drastic increase of its incidence linked to PSA screening (400,000 new cases in Europe per year of which 70,000 in France). Most cancers are now detected at an early stage and a large number of patients have cancers whose 10-year risk of metastasis spreading is low or moderate.
Currently those patients are given radical treatment (with the risk of urinary and sexual side effects) or are placed under active monitoring (also known as surveillance) with a delayed treatment in case of progression (with the risk, in some cases, of achieving treatment too late as shown by the UK study “Protect”). The main goal of HIFU is to provide an alternative to both the radical treatment and the active monitoring of patients whose tumors were properly -assessed through combination of multiparametric MRI and imaging-guided biopsies. Post-treatment MR-imaging allows for accurately assessing the extent of the destroyed area. More recently though, enhanced contrast ultrasound has enabled peroperative control of treatment efficacy, allowing the operator, if necessary, to carry out an immediate completion of the therapy.
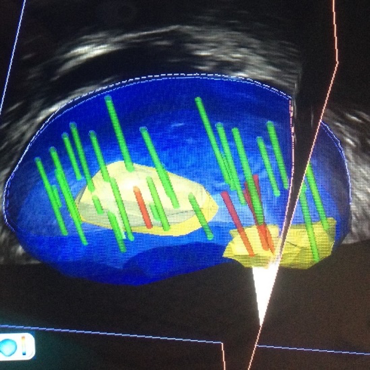
HIFU treatment is a treatment that can be performed under locoregional anesthesia with a 24-hour hospitalization. HIFU are delivered transrectally by a firing probe that combines a therapy transducer (which transmits successive 1-second bursts of HIFU) and an US-imaging transducer (which allows the prostate to be located in real time during the entire treatment). At the focus of the ultrasonic beam, the absorption of energy creates a temperature rise between 85°C and 100°C which induces an irreversible ablation of the cancerous tissue (coagulative necrosis). By repeating the HIFU bursts and by moving the firing head, it is possible to ablate a very precise volume. Since it is not ionizing radiation, treatment can be repeated if necessary, or used as a salvage treatment for patients with local recurrence after undergoing radiotherapy. The latest-generation HIFU device (Focal One ™, Figure 1) allows, when the tumor only invades part of the gland, to limit the treatment to the cancer area (so-called focal treatment) and to preserve the non-cancerous prostatic tissues. As such, the risk of digestive urinary and sexual side effects is then considerably reduced compared to conventional radical treatment (radical prostatectomy or external radiotherapy). To guide the treatment, the operator uses an image fusion software that allows for visualization of the tumor on the screen of the device as it appears on the preoperative MRI as well as the biopsies shown in 3D imaging (Figure 2).
The project, started in 1990, resulted in the marketing of 3 successive machines: Ablatherm Maxis in 2000, Ablatherm Integrated Imaging in 2005 and Focal.One in 2013. HIFU treatment is now available in most countries around the world except for Japan. More than 50,000 treatments have been performed to date.
The French Association of Urology (AFU) has been the promoter of 3 multicenter French studies: the latest, carried out in the legal framework of the innovation package, is a comparative study with radical surgery concerning both first-line and recurrent patients after radiotherapy. Regarding the focal treatment, there are currently 3 ongoing studies as part of a Research Hospital University (RHU) project funded by the ANR.
Figure 1 : FocalOne ™ device.

Figure 2 : 3D imaging of MRI / biopsies fusion.
Staff
Sébastien Crouzet
Olivier Rouvière
Cyril Lafon
Rémi Souchon
Stéfan Catheline
Jorge Torres
Publications
Khedime S, Gelet A, Rouvière O, Lafon C, Badet L, Crouzet S, Hostiou T.
Salvage High-Intensity Focused Ultrasound for Local Recurrence in the Prostatic Bed after Prostatectomy and Adjuvant or Salvage Radiotherapy: Preliminary Results.
J Urol. 2021 Aug;206(2):325-337. doi: 10.1097/JU.0000000000001771.
Payen T, Crouzet S, Guillen N, Chen Y, Chapelon JY, Lafon C, Catheline S.
Passive Elastography for Clinical HIFU Lesion Detection.
IEEE Trans Med Imaging. 2024 Apr;43(4):1594-1604. doi: 10.1109/TMI.2023.3344182.
Torres J, Accary V, Payen T, Delattre V, Lafon C.
Acoustic focal characteristics of a transrectal phased array under heterogeneity and weak nonlinearity.
J Acoust Soc Am. 2025 Nov 1;158(5):4146-4158. doi: 10.1121/10.0039947.




