HIFU Liver Pancreas
Project Description
Surgical resection is the mainstay of treatment for many cancers. However, for several tumor types (i.e. primary pancreatic tumor, liver metastases…) most of the patients are ineligible for surgical resection; furthermore, surgery may have severe risks for the patient, depending on tumor type and location. During the last few decades, there has been widespread interest in the development and refinement of ablation techniques for local treatment of tumors. The development of imaging modalities and therapeutic devices has made image-guided tumor ablation a clinical and commercial reality. Image-guided tumor ablation using High Intensity Focused Ultrasound (HIFU) is now an attractive treatment option, for curative as well as for palliative treatment. We have developed a toroidal HIFU transducerenabling destruction of large liver volumes within a short period of time. The device has been tested in vivo on pig and rabbit models. Using this technology there is no need to puncture the parenchyma, the extent of the thermal lesions achieved is not reduced by hepatic perfusion, and it is possible to monitor the therapeutic effect with conventional ultrasound imaging. Such preclinical work is now translated into clinical practice through controlled trials to assess the feasibility and safety of HIFU ablation in patients with colorectal liver metastases (Phase I and IIaclinicial trials have been completed, phase IIb trial is on the way to be closed). A surgical device that uses this technology will be adapted to the treatment of non resectable pancreatic tumors. This disease is the 5th cause of death from digestive cancer, with increasing incidence. Surgery is the standard treatment in 10-15% of the patients. It is frequently contra indicated because of an advanced stage (locally advanced or metastatic disease). This lack of efficient therapeutics explains the need of alternative techniques. The promising results obtained in treating liver metastases from colorectal cancer using HIFU have encouraged us to adapt the medical device for treating pancreatic tumors. Based on the toroidal transducer described previously a new device has been developed. Preclinical studies in pigs have demonstrated efficacy to destroy large volume of pancreas. These results allows tostart a first in man phase I-II clinical trial to assess HIFU ablation in patients with locally advanced non-metastatic pancreas cancer being not resectable after induction chemotherapy.
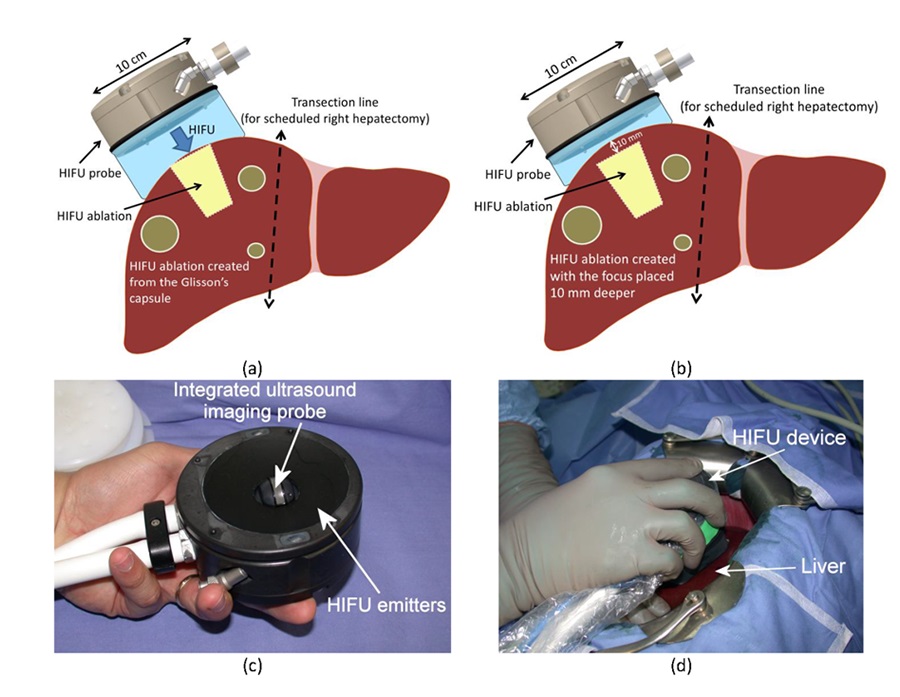
HIFU Probe and schematic views of the ablated area. (a) Schematic diagram of a HIFU treatment with the HIFU focus placed in the liver such asHIFU ablations extent from the Glisson’s capsule. (b) Using less water in the cooling envelope the focus can be placed deeper in tissues (10 mm) such asHIFU ablations can be created deeper in the liver. (c) Photograph of the HIFU device. (d) Intraoperative view of the HIFU device. 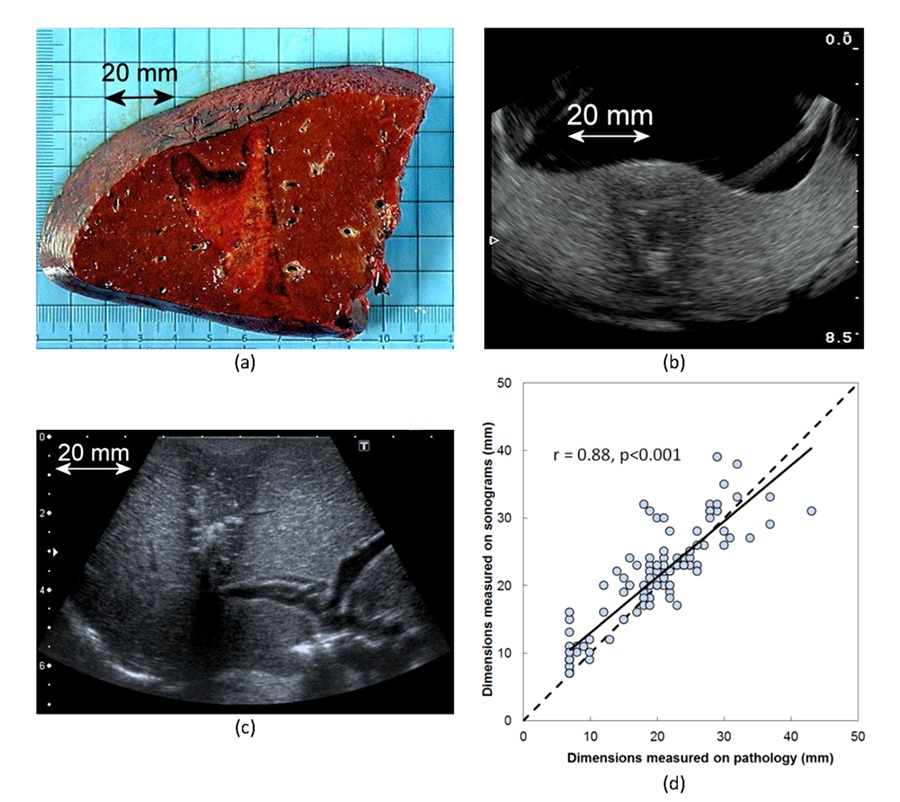
Macroscopic and echographicvisualzation of HIFU ablated area. HIFU ablations were clearly visible in ultrasound images. Dimensions measuredon sonograms were correlated to the dimensions measured on gross pathology. (a) Typical HIFU ablation observed on gross pathology, (b) on sonogramsobtained with the integrated ultrasound imaging probe (IUSP) of the HIFU device and (c) on sonograms obtained with a higher resolution ultrasound imagingprobe. (d) Correlation between dimensions measured on sonograms using IUSP and gross pathology.
Funding
- BPI, HECAM :HEpatocellularCArcinoma Multi-technological - 967 k€ / 18 M€ - 01/2015 à 12/2019
- PHRC, Randomized, open-label, phase II study to evaluate High-Intensity Focused Ultrasound Assisted Hepatic Resection (HIFU-AR) during a liver resection on humans with a focus on evaluation of blood loss and safety – 146 k€ - 09/2012 à 09/2016
- INCa, LYRIC: LYon Recherche Intégrée en Cancérologie – 668 k€ / 10.7 M€ - 01/2012 à 01/2017
- PHRC, Evaluation, chez des patients nécessitant une chirurgie de résection de métastases hépatiques de cancers colorectaux, l’utilisation per-opératoire d’HIFU en termes de faisabilité, d’innocuité, et de capacité de ciblage des métastases – 150 k€ - 06/2010 à 06/2016
- Cancéropôle Lyon Auvergne Rhône Alpes dans le cadre du programme « Preuve du Concept » (PDC 2006.4.8), High IntensityFocusedUltrasound for the treatment of livermetastases – 530 k€ - 02/2007 à 12/2013
- ARC, Miniaturisation d’un dispositif médical HIFU pour le traitement des métastases hépatiques – 50 k€ - 12/2006 à 12/2009
Staff
LabTau Staff
- David Melodelima (DR INSERM, PI)
- Michel Rivoire (PU-PH CLB, co-PI)
- Aurélien Dupré (PH CLB)
- Yao Chen (Surgeon CLB)
- Stefan Langonnet (Veterinary CLB)
Collaborations
- Department of Surgery of the Centre Leon Berard
- EDAP - TMS
Publications
- Dupré A, Pérol D, Blanc E, Peyrat P, Basso V, Chen Y, Vincenot J, Kocot A, Melodelima D, Rivoire M. Efficacy of high-intensity focused ultrasound-assisted hepatic resection (HIFU-AR) on blood loss reduction in patients with liver metastases requiring hepatectomy: study protocol for a randomized controlled trial.
- Dupré A, Melodelima D, Pflieger H, Chen Y, Vincenot J, Kocot A, Langonnet S, Rivoire M. Thermal Ablation of the Pancreas With Intraoperative High-Intensity Focused Ultrasound: Safety and Efficacy in a Porcine Model.
- N'Djin WA, Chapelon JY, Melodelima D. An Ultrasound Image-Based Dynamic Fusion Modeling Method for Predicting the Quantitative Impact of In Vivo Liver Motion on Intraoperative HIFU Therapies: Investigations in a Porcine Model.
- Dupré A, Melodelima D, Pérol D, Chen Y, Vincenot J, Chapelon JY, Rivoire M. First clinical experience of intra-operative high intensity focused ultrasound in patients with colorectal liver metastases: a phase I-IIa study.
- Vincenot J, Melodelima D, Chavrier F, Vignot A, Kocot A, Chapelon JY. Electronic beam steering used with a toroidal HIFU transducer substantially increases the coagulated volume.
- Pichardo S, Kivinen J, Melodelima D, Curiel L. Suitability of a tumour-mimicking material for the evaluation of high-intensity focused ultrasound ablation under magnetic resonance guidance.
- Melodelima D, Chenot J, Souchon R, Rivoire M, Chapelon JY. Visualisation of liver tumours using hand-held real-time strain imaging: results of animal experiments.
- Gandini A, Melodelima D, Schenone F, N'Djin AW, Chapelon JY, Rivoire M. High-intensity focused ultrasound (HIFU)-assisted hepatic resection in an animal model.
- N'Djin WA, Melodelima D, Schenone F, Rivoire M, Chapelon JY. Assisted hepatic resection using a toroidal HIFU device: an in vivo comparative study in pig.
- Chenot J, Melodelima D, N'djin WA, Souchon R, Rivoire M, Chapelon JY. Intra-operative ultrasound hand-held strain imaging for the visualization of ablations produced in the liver with a toroidal HIFU transducer: first in vivo results.
- N'Djin WA, Melodelima D, Parmentier H, Rivoire M, Chapelon JY. In vivo preclinical evaluation of the accuracy of toroidal-shaped HIFU treatments using a tumor-mimic model.
- Melodelima D, N'Djin WA, Favre-Cabrera J, Parmentier H, Rivoire M, Chapelon JY. Thermal ablation produced using a surgical toroidal high-intensity focused ultrasound device is independent from hepatic inflow occlusion.
- Parmentier H, Melodelima D, N'Djin A, Chesnais S, Chapelon JY, Rivoire M. High-intensity focused ultrasound ablation for the treatment of colorectal liver metastases during an open procedure: study on the pig.
- Melodelima D, N'Djin WA, Parmentier H, Chesnais S, Rivoire M, Chapelon JY. Thermal ablation by high-intensity-focused ultrasound using a toroid transducer increases the coagulated volume. Results of animal experiments.
- N'Djin WA, Melodelima D, Parmentier H, Chesnais S, Rivoire M, Chapelon JY. Utility of a tumor-mimic model for the evaluation of the accuracy of HIFU treatments. results of in vitro experiments in the liver.




