Bone metastases
Project Description
LEUS-drug interactions: LEUS + bisphosphonate and doxorubicin for the treatment of bone metastases; extension to primary breast tumors
Ultrasound (US) is a non-ionizing pressure wave that can produce mechanical and thermal effects. Bisphosphonates have demonstrated clinical utility in bone metastases treatment. Preclinical studies suggest that bisphosphonates have anticancer activity. However, bisphosphonates exhibit a high affinity for bone mineral, which reduces their bioavailibityfor tumor cells. Ultrasound has been shown to be effective for drug delivery but in interaction with gas bubbles or encapsulated drugs. We examined the effects of a clinically relevant dose of bisphosphonate zoledronate (ZOL) in combination with US. In a bone metastasis model, mice treated with ZOL+US had osteolytic lesions that were 58% smaller than those of ZOL-treated animals as well as a reduced skeletal tumor burden. In a model of primary tumors, ZOL+US treatment reduced by 42% the tumor volume, compared with ZOL-treated animals. Using a fluorescent bisphosphonate, we demonstrated that US forced the release of bisphosphonate from the bone surface, enabling a continuous impregnation of the bone marrow. Additionally, US forced the penetration of ZOL within tumors, as demonstrated by the intratumoralaccumulation of unprenylated Rap1A, a surrogate marker of ZOL antitumor activity. Our results demonstrate the potential of low intensity ultrasound as an effective strategy to force bisphosphonate desorption from bone and its penetration through tumor tissue, enabling bisphosphonate antitumor activity (both in bone and outside bone). Our findings made US a promising modality in oncology to trigger anticancer therapy with bisphosphonates.
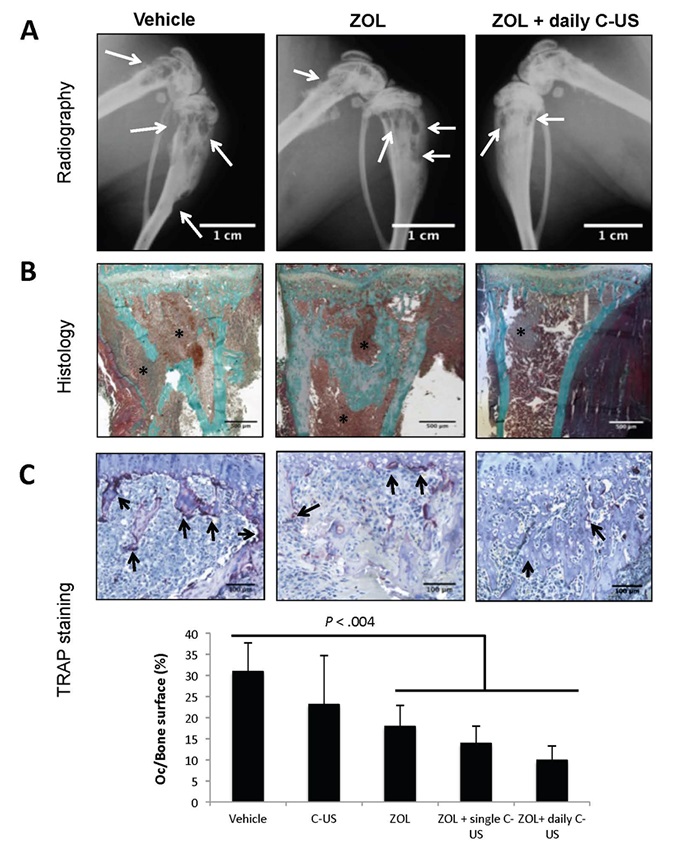
Effects of zoledronate (ZOL), alone or in combination with low-intensity continuous ultrasound (US), on the progression of established breast cancer bone metastases. (a) Radiographic analysis of hind limbs from B02 tumor-bearing mice treated with the vehicle, ZOL or the combination of ZOL with US. Arrows indicate osteolytic lesions. (b) Goldner’s trichrome staining of tissue sections of tibial metaphysis from metastatic legs. Bone is stained green whereas bone marrow and tumor cells (asterisk) are stained brown. (c) upper panels: tartrate-resistant acid phosphatase (TRAP) staining of bone tissue sections of metastatic legs from mice, showing osteoclast resorption surfaces (arrows). Bottom graph: Osteoclast resorption surface was calculated as the ratio of TRAP-positive trabecular bone surface to the total trabecular bone surface at the tumor-bone interface. All images were obtained from different mice on day 32 after tumor cell inoculation. The images shown are examples that best illustrate the effects of the different treatments on bone metastasis formation.
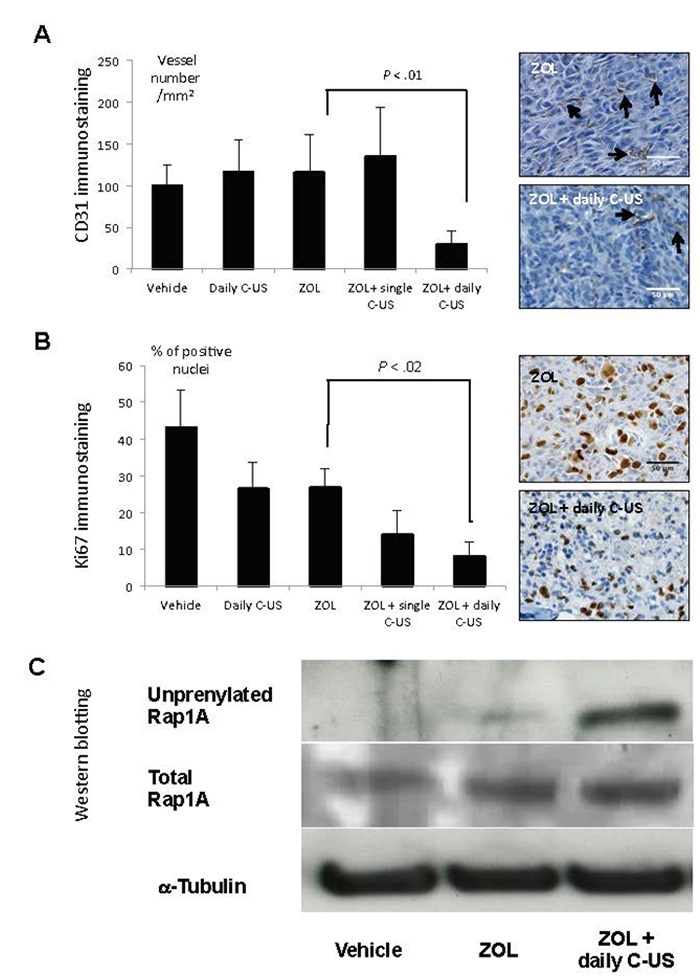
Effects of zoledronate (ZOL), alone or in combination with low-intensity continuous ultrasound (US), on B02 skeletal tumor burden. (a) Quantification of CD31-positive blood vessels within skeletal tumors. Right-hand panels: immunostaining of CD31-positive blood vessels (arrows) within skeletal tumors from animals treated with ZOL or ZOL + daily US. (b) Quantification of tumor-cell proliferation, as judged by the percentage of Ki-67–positive nuclei. Right-hand panels: Ki-67 nuclear antigen immunostaining within skeletal tumors from animals treated with ZOL or ZOL + daily US. Proliferative cells are stained brown. (c) Immunodetection of unprenylated and total Rap1A in protein extracts from skeletal tumors of animals treated with the vehicle, ZOL or ZOL+US. Cropped gels are presented. Full-length blots are presented in Supplementary Figure 6. Tubulin was used as a control for equal protein loading. All data were obtained from different mice on day 32 after tumor cell inoculation. The images shown are examples that best illustrate the effects of the different treatments on bone metastasis formation.
Funding sources
- SATT LUTECH (LRTCA)
- ANR T-ERC 2016 (LabTAU, INSERM)
Staff
LabTau Staff
- PIs: David MELODELIMA (DR INSERM)
- co-PIs: Philippe CLEZARDIN (DR INSERM, Directeur du Lyos)
- Students: Sophie TARDOSKI (Doctorante), IshmaèneBENZAÏD (Postdoctorante), Jacqueline NGO (Ingénieure)
Collaborations
- Research laboratory in advanced surgical technologies (LRTCA) team: http://www.lrtca.org/
- Infantile Epilepsy and Brain Plasticity (INSERM U1129) team: http://www.idf.inserm.fr/rubriques/les-laboratoires/implantations/structures-de-recherche-paris-5/annexes/umr-1129
Publications
- RAS




